Past clinical trials in ovarian cancer
Through analysis of thousands of patients in clinical trials around the world, we have developed new ways of detecting and monitoring ovarian cancer based on changes in levels of CA125 – a chemical present in the blood of patients with ovarian cancer. These tests also provide an early indication of patients’ responses to different treatments. Our definitions, based on changes in CA125 levels (previously known as the “Rustin” criteria), have now been accepted by all major trial groups around the world and are called the Gynecological Cancer Inter-Group (GCIG) criteria. The CTRT helped fund the CA125 doubling trial that demonstrated that CA125 changes could be used to assess new drugs which are relatively non-toxic and work by controlling rather than killing cancers (Hall et al., 2014).
Another major area of development supported by CTRT and undertaken at Mount Vernon Cancer Centre over the last 20 years, involves a group of drugs that attack the blood vessels which prevents ovarian cancer from growing. The CTRT has contributed to the necessary research teams that allow our patients access the clinical trials with the anti-angiogenic agents (ICON 7, ICON6, AMG386). These agents have been shown to prolong remissions in patients with persistent and relapsed ovarian cancer (Hall, 2019; Oza, 2015; Perren, 2011). Professor Hall was the chief investigator that headed the national ‘METRO-BIBF’ clinical trial. She explored another anti-angiogenic called nintedanib, with oral cyclophosphamide (Hall et al., 2020). Although the addition of nintedanib did not lengthen the time in remission for patients in this study, it was useful in highlighting the value of oral cyclophosphamide in this patient group. The repurposing of older drugs such as cyclophosphamide is becoming increasingly valuable. At CTRT we hope that future clinical trials can help us explore the combination of cyclophosphamide with alternative agents, which may induce longer and more successful remissions for these patients.
The second group of drugs to target the blood vessels are vascular disruptive agents (VDAs). Professor Rustin was an instrumental figure in the early development of two of these drugs, combretastatin and fozbretabulin. He was the Chief Investigator of the PAZ-FOZ trial in patients with recurrent ovarian cancer, this investigated combining a vascular disruptive agent (fozbretabulin) with an anti-angiogenic (Pazopanib) – the drugs that attack tumour blood vessels in very different ways (see figure 1). Sadly, the combination caused too many cardiac problems for widespread development, although the principle of this combination was very successful (Morgan et al., 2020).
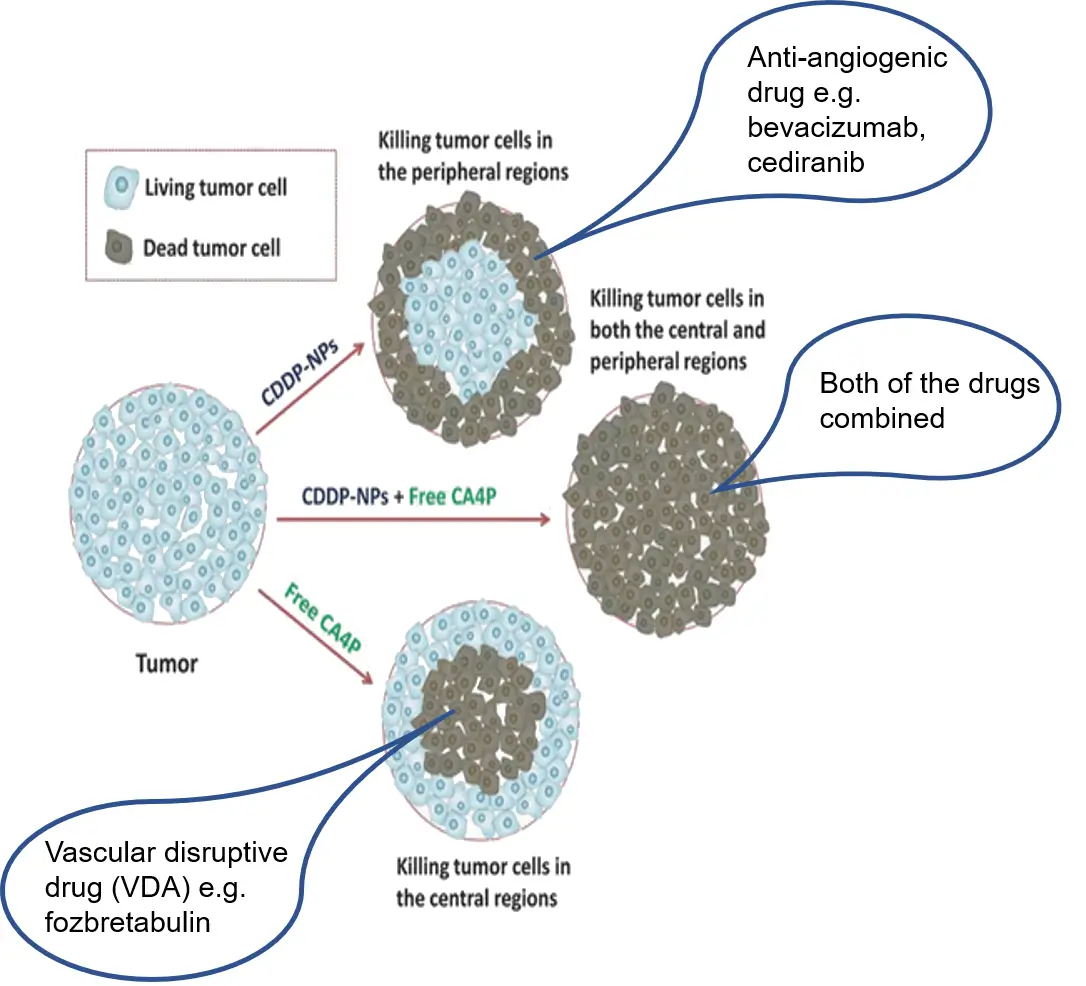
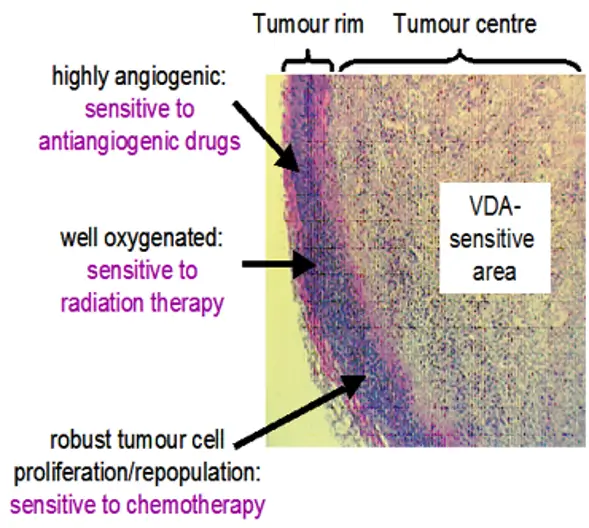
Comparison of the action of antiangiogenic and vascular disruptive agents.
There was an article in the Daily Mail (14 October 2014) regarding this trial – Is this the Ovarian Cancer Breakthrough Women have Prayed For?
In the laboratory
CTRT funds the tuition and bench fees for a clinical research fellow who is in collaboration with Dr Emmanouil Karteris.
Dr Emmanouil Karteris is a Senior Lecturer at Brunel University (Laboratory of Cancer Biomarkers and Cellular Endocrinology) and is exploring the circulating cancer-associated cells within patients that have ovarian and uterine cancer. Her PhD thesis also integrates CT scan imagery that shows the changes in some ovarian cancer patients with molecular findings from their blood samples. To predict which therapies might be most effective depends on the ability to identify novel imaging markers from the molecular findings.

PhD students, clinical research fellow and scientific officers with laboratory head Dr Emmanouil Karteris and Professor Marcia Hall (centre)
Bibliography
- Carter, T., Jeyaneethi, J., Kumar, J., Karteris, E., Glynne-Jones, R. and Hall, M., 2020. Identification of Cancer-Associated Circulating Cells in Anal Cancer Patients. Cancers, 12(8), p.2229. DOI: 10.3390/cancers12082229
- Chudasama, D., Bo, V., Hall, M., Anikin, V., Jeyaneethi, J., Gregory, J., Pados, G., Tucker, A., Harvey, A., Pink, R. and Karteris, E., 2017. Identification of cancer biomarkers of prognostic value using specific gene regulatory networks (GRN): a novel role of RAD51AP1 for ovarian and lung cancers. Carcinogenesis, 39(3), pp.407-417. DOI: 10.1093/carcin/bgx122
- Hall, M., Petruckevitch, A., Pascoe, J., Persic, M., Tahir, S., Morgan, J., Gourley, C., Stuart, N., Crawford, S., Kornbrot, D., Qian, W. and Rustin, G., 2014. Using Serum CA125 to Assess the Activity of Potential Cytostatic Agents in Ovarian Cancer. International Journal of Gynecologic Cancer, 24(4), pp.676-681. DOI: 10.1097/igc.0000000000000116
- Hall, M., Bertelli, G., Li, L., Green, C., Chan, S., Yeoh, C., Hasan, J., Jones, R., Ograbek, A. and Perren, T., 2019. Role of front-line bevacizumab in advanced ovarian cancer: the OSCAR study. International Journal of Gynecologic Cancer, 30(2), pp.213-220. DOI: 10.1136/ijgc-2019-000512
- Hall, M., Dehbi, H., Banerjee, S., Lord, R., Clamp, A., Ledermann, J., Nicum, S., Lilleywhite, R., Bowen, R., Michael, A., Feeney, A., Glasspool, R., Hackshaw, A. and Rustin, G., 2020. A phase II randomised, placebo-controlled trial of low dose (metronomic) cyclophosphamide and nintedanib (BIBF1120) in advanced ovarian, fallopian tube or primary peritoneal cancer. Gynecologic Oncology, 159(3), pp.692-698. DOI: 10.1016/j.ygyno.2020.09.048.
- Kumar, J., Chudasama, D., Roberts, C., Kubista, M., Sjöback, R., Chatterjee, J., Anikin, V., Karteris, E. and Hall, M., 2019. Detection of Abundant Non-Haematopoietic Circulating Cancer-Related Cells in Patients with Advanced Epithelial Ovarian Cancer. Cells, 8(7), p.732. DOI:10.3390/cells8070732
- Morgan, R., Banerjee, S., Hall, M., Clamp, A., Zhou, C., Hasan, J., Orbegoso, C., Taylor, S., Tugwood, J., Lyon, A., Dive, C., Rustin, G. and Jayson, G., 2020. Pazopanib and Fosbretabulin in recurrent ovarian cancer (PAZOFOS): A multi-centre, phase 1b and open-label, randomised phase 2 trial. Gynecologic Oncology, 156(3), pp.545-551. DOI: 10.1016/j.ygyno.2020.01.005
- Oza, A., Cook, A., Pfisterer, J., Embleton, A., Ledermann, J., Pujade-Lauraine, E., Kristensen, G., Carey, M., Beale, P., Cervantes, A., Park-Simon, T., Rustin, G., Joly, F., Mirza, M., Plante, M., Quinn, M., Poveda, A., Jayson, G., Stark, D., Swart, A., Farrelly, L., Kaplan, R., Parmar, M. and Perren, T., 2015. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. The Lancet Oncology, 16(8), pp.928-936. DOI: 10.1016/S1470-2045(15)00086-8.
- Perren, T., Swart, A., Pfisterer, J., Ledermann, J., Pujade-Lauraine, E., Kristensen, G., Carey, M., Beale, P., Cervantes, A., Kurzeder, C., Bois, A., Sehouli, J., Kimmig, R., Stähle, A., Collinson, F., Essapen, S., Gourley, C., Lortholary, A., Selle, F., Mirza, M., Leminen, A., Plante, M., Stark, D., Qian, W., Parmar, M. and Oza, A., 2011. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. New England Journal of Medicine, 365(26), pp.2484-2496. DOI: 10.1056/NEJMoa1103799
- Saravi, S., Katsuta, E., Jeyaneethi, J., Amin, H., Kaspar, M., Takabe, K., Pados, G., Drenos, F., Hall, M. and Karteris, E., 2020. H2A Histone Family Member X (H2AX) Is Upregulated in Ovarian Cancer and Demonstrates Utility as a Prognostic Biomarker in Terms of Overall Survival. Journal of Clinical Medicine, 9(9), p.2844. DOI: 10.3390/jcm9092844
Relates Posts
Professor Gordon Rustin – Founder of The Cancer Treatment and Research Trust
Professor Gordon Rustin (MBBS, MD, MSc, FRCP) founded The Cancer Treatment and Research Trust in 1985 whilst working as a Senior Lecturer in Medical Oncology at Charing Cross Hospital. He continued his role of Chair at CTRT when he moved full-time to Mount Vernon Hospital where he became Director of Medical Oncology in 1995 leading research in the management of gynaecological cancers, germ cell tumours and the use of tumour markers and clinical trials in vascular disruptive agents, resulting in more than 350 publications.
He has now retired from research and consulting but is still an active and vital member of the CTRT trustee team. Gordon has taken the time to tell us more about his fascinating career, how he set up CTRT, his fundamental work in cancer research and how he sees CTRT’s role in future cancer research.
Why and how did you start your career in Oncology?
I was a general medical registrar undertaking varied medical specialities from neurology to chest medicine, until I decided on oncology as a medical career. I thoroughly enjoyed cardiology and in particular interventional radiology, but as I am tall and thin, I didn’t think my back would cope with 40 years of wearing lead aprons! I realised that oncology was the area in my lifetime where I would see the greatest advancements in medicine which I found truly exciting so decided to pursue this avenue.
I received a research fellowship at the Hammersmith Hospital where I worked hard and obtained my MD thesis and published several papers on why neutrophil alkaline phosphatase is elevated in a type of leukaemia. I then was assigned a post at the Charing Cross Hospital and have been in medical oncology ever since. I was made a consultant and senior lecturer in medical oncology at the Charing Cross Hospital in 1984. Then in 1995, I moved full time to Mount Vernon Hospital to become director of Medical Oncology and became the first site specialist oncologist there concentrating on managing patients with testicular and ovarian cancers. I also led research and clinical trials of vascular disruptive agents, and circulating tumour markers, especially CA125.
Why did you decide to set up CTRT?


Whilst I was a medical consultant at Charing Cross in the 80s, I was a senior lecturer and employed by the university in an academic post. The Imperial College rules at that time were that if you earnt more than 10% of your university salary in private practice anything over that went to the university. I was at that time co-directing the Trophoblast unit (Michael Seckl a fellow CTRT trustee, took over my post when I moved to Mount Vernon), which is the most highly rated units in the world for treatment of cancer of the placenta. The fees from treating just one private patient were more than 10% of my university salary. A friend of mine, who is a lawyer, suggested that we set up a charity to direct where this money would go. Myself and two colleagues, Richard Begent who became Professor of Oncology at the Royal Free Hospital and Ed Newlands who was given a personal chair at Charing Cross Hospital, set up CTRT and within about 3 or 4 years we were remarkably bringing in a substantial amount of money for research, not just from our private patients but increasingly from donations from friends and family of our patients and from pharmaceutical companies. This had huge ramifications for our work. Through the charity, myself and my colleagues were able to free up time for more consultations and clinical research. The whole infrastructure in fact changed for the better because of CTRT. Instead of spending months each year writing grant applications we spent just a few hours each month running CTRT. This gave us the time to devote to research and the funds to appoint research nurses, data managers, laboratory scientists and equipment. It made a huge difference and brought enormous momentum to the research units, and we are still reaping the benefits today.
What has been the most significant study that you have been involved in within your career that makes you proud?
There have been two areas that with the help of CTRT have been very significant in my career.
The first one is research into circulating tumour markers. (These are markers of cancer that can be detected in the body including in blood, urine & saliva) We had been looking for ways of monitoring ovarian cancer, which is known as the ‘silent cancer’ as it is very difficult to detect and monitor. We needed a method to monitor patients, without the need for scanning them on a regular basis, so we started looking into various blood tests to do this. We had already done an extensive amount of work on tumour markers in trophoblast disease such as choriocarcinoma disease using the tumour marker HCG and had showed the value of monitoring HCG and AFP markers in managing testicular cancer. Together with colleagues at the Hammersmith Hospital we started clinical trials into ovarian cancer which included regular blood sampling for tumour markers. These sample banks were crucial for testing new tumour markers for ovarian cancer. Once we could see that CA125 was a promising tumour marker for ovarian cancer I proposed various criteria that could be used to indicate whether the cancer was responding to treatment, or the cancer was relapsing. CTRT funded the research nurses, laboratory technicians, data managers and statisticians who collected the samples, ran the assays and analysed the results. Having produced criteria for defining response and progression, with funding from CTRT I managed to get data from most of the major trial groups around the world to test these criteria.
We obtained serial CA125 results from over 5,000 ovarian cancer patients worldwide who were taking part in clinical trials, and through this we were able to improve and validate our definitions that defined whether patients were responding to treatment. After several publications on these criteria, they were accepted internationally and became known as the ‘Rustin Criteria’. Later after I chaired an international committee of the Gynaecological Cancer Intergroup (GCIG), they were formalised as the ‘GCIG Criteria’. This became internationally recognised and is still used in clinical trials today.

Following that, we became aware that patients who had been treated for ovarian cancer, often experienced a rise in CA125 levels weeks, months or even years before they developed symptoms of relapsing ovarian cancer. There was a big debate at the time into whether patients should restart chemotherapy as soon as their CA125 levels started to rise, or if we should wait until they presented with symptoms. I set up an international trial run by the Medical Research Council (MRC) to see what was the best approach, and again CTRT contributed funding so that we had the staff to enter almost a hundred patients into this trial. Patients were randomised once their CA125 levels started rising to either receiving immediate treatment or waiting until symptoms occurred. It was a blinded trial in which 1442 patients entered and 529 were randomised to either immediate or delayed treatment of relapse. In 2009 we submitted the results to the American Society of Clinical Oncologists, the largest cancer meeting in the world and it was accepted as the most important paper in the plenary session. I presented the data to 15,000 people and I still to this day remember the gasp of surprise and horror when I was able to present the data that showed that those who had received immediate treatment didn’t live a day longer than those that received delayed treatment. It made a huge impact in terms of deciding treatment options for cancer patients at the time, which was very exciting to be a part of!
The other area of research that is most significant to me arose from a discussion I had in 1987 with Juliana Denenkamp who at the time was director of the Grey laboratory at Mount Vernon Hospital. She was very involved in trying to work out how we could make radiotherapy more effective for cancer patients. It was clear at the time that radiotherapy was not effective in large cancer tumours as the cells in the middle were not being killed. For radiotherapy to be effective, it must react with oxygen and the centre of the large cancer tumours have little or no oxygen. Juliana showed me the results from an experiment using a chemical flavone acetic acid that they had received from a lab in Auckland, New Zealand which they were hoping would increase blood flow and it did the exact opposite. In fact, within 45 minutes the tumour masses were just dead lumps of tissue. This was no value to them but could be for us! So, with support from Cancer Research UK and funding from CTRT we started undertaking clinical trials with a series of vascular disruptive agents on patients with large untreatable cancer tumours. We used dynamic MRI scanning so we could measure blood flow through the tumours in real time which no one had done before. We have gained an international reputation for excellence in this area as we have carried out more trials in vascular disruptive agents at the Mount Vernon Cancer Centre than any other cancer centre in the world.
How impactful has funding from CTRT been on your ability to carry out your research over the years?
To conduct clinical research you need research staff and equipment and you need to source the funds for that. I was lucky that I was able to find some excellent people to assist in our cancer research studies and CTRT made a major contribution to the funding of their posts. One of the most important aspects that CTRT has brought to the forefront for clinical research is ‘time’. Consultants need time to do research and the NHS requires consultants to have ‘protected research sessions’ in which to conduct their studies and CTRT has been able to facilitate that through funding these sessions.
How has funding from CTRT contributed to the advances in cancer research you were able to make during your career?
CTRT was and still is fundamental in advancing cancer treatments. We wouldn’t have been able to have carried out a tumour marker clinical trial on ovarian cancer for instance without funding from CTRT. It is important to know whether a cancer is growing or shrinking, and it was thanks to CTRT that we could better define what is happening to cancer in this instance and whether treatment is working. On the testicular cancer side, you need databases of patients in order to analyse samples and test results etc and so thanks to CTRT, we were able to fund data managers who collated thousands of patient’s data to use for trials. We were very involved in proving that patients with early stages of testicular cancer did not require any chemotherapy and could just be monitored. The cure rate of those patients being monitored were just as high or even higher than those receiving chemotherapy. In terms of our vascular disruptive therapy research, treatment using drugs that target blood vessels discovered during our research have made a big impact in the management of many different cancers. Although pharmaceutical companies and CRUK and other major charities such as the National Lottery made major contributions, without CTRT there would not have been sufficient research staff to perform these trials.
How do you envision the future for cancer treatment and research, and CTRT’s role within this?


The major cancer charities such as CRUK as well as pharmaceutical companies make a large contribution to cancer research through the discovery of new drugs. However, when it comes to conducting clinical research there are always some areas that are not adequately funded to be able to produce good clinical data for effective treatments to be discovered. Clinical trials need many qualified staff to run the studies including research nurses and data managers, as well as consultants and that’s where I see CTRT making that difference. CTRT has, and continues to, fund research into studies of lesser known and rare types of cancers which receive less funding from elsewhere. With the help from CTRT, we are able to fund vital medical research staff that make it all happen for cancer patients and future cancer research developments.

Immunotherapy treatment discovered that can have long-term survival benefit for Uveal melanoma
At CTRT, we are dedicated to finding better ways of treating cancer, as well as detecting, monitoring and understanding how cancer develops, particularly in rare cancers that don’t receive research funding elsewhere.
One of our trustees, Professor Paul Nathan has led a clinical trial at the Mount Vernon Cancer Centre, and has discovered a more successful way of treating Uveal melanoma.
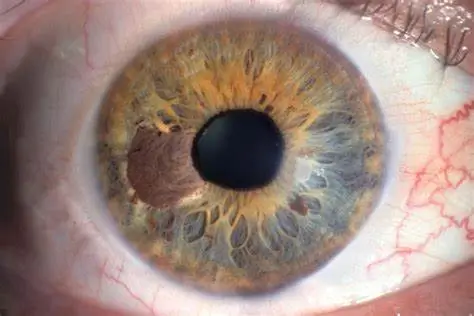
Uveal melanoma is a rare, but often high risk, type of cancer of the eye that frequently spreads to other parts of the body (particularly the liver). Unlike skin melanoma, once the cancer has spread, it does not respond very well to so-called immune checkpoint inhibitors, a type of cancer treatment that uses the body’s own immune system to recognize and fight off cancer cells. However, data from the clinical trial led by Professor Paul Nathan at the Mount Vernon Cancer Centre indicated that another type of immunotherapy can have long-term survival benefit in patients with metastatic uveal melanoma, a form of the disease that has spread.
The immunotherapy used in the trial, tebentafusp, works by bringing cells of the immune system close to the melanoma cells, effectively directing the immune cells to kill the cancer cells. Tebentafusp can perform this bridging role because it was designed as a ‘bispecific fusion protein’, which means that it latches on to pieces of a particular protein that melanoma cells have in abundance on their surface, as well as to a protein called CD3, that is present on T cells, a type of immune cell that is particularly efficient at killing cancer cells.
Initial reports showed that, at 1 year, tebentafusp improved the survival of patients with metastatic uveal melanoma, and the latest trial results which have been published recently in the New England Journal of Medicine, showed that the benefit is still seen at 3 years.

Nearly 400 patients, all of whom had previously untreated metastatic uveal melanoma, took part in the trial. They were randomly assigned to receive either tebentafusp or another treatment — a checkpoint inhibitor (pembrolizumab or ipilimumab) or chemotherapy (dacarbazine). At 3 years, more of the patients who had been given tebentafusp were still alive (27%) compared with the patients receiving any of the other treatments (18%). The side effects of tebentafusp were similar to those seen previously which included a rash, fever/chills, itching and low blood pressure. Very few patients receiving any of the drugs had to stop treatment, and there were no treatment-related deaths during the trial.
Tebentafusp clearly presents an advantage over current treatments for Uveal melanoma but unfortunately, it does not offer a cure at present. Moreover, tebentafusp only works in a subset of patients — making broadening tebentafusp’s potential benefit another priority.
Nevertheless, the future for tebentafusp, perhaps in combination with other treatments, looks very promising. Professor Nathan is leading an international trial due to begin in summer 2024 that will look at whether tebentafusp, if given after the primary treatment to tackle the melanoma in the eye, can reduce the number of patients who relapse with metastatic disease. This clinical trial will take place at many centres throughout Europe and North America and is being run by the European Organisation for Research and Treatment of Cancer.
The trial was run and funded by Immunocore, with some staffing costs at Mount Vernon Hospital supported by CTRT.
The Cancer Treatment and Research Trust continues to support Professor Nathan’s research thanks to your generosity. You can donate towards our melanoma research here
Research paper published in the New England Journal December 2023: Hassel, J.C. Three-year overall survival with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2023 Dec 14;389(24):2256-2266. doi: 10.1056/NEJMoa2304753.