Lung Cancer
Lung Cancer
Lung cancer is a malignant tumour that begins when abnormal cells grow uncontrollably in the lungs. It is the leading cause of cancer deaths worldwide, primarily affecting individuals who smoke, although non-smokers can also develop the disease. Symptoms may include a persistent cough, chest pain, and shortness of breath.
Unfortunately, most lung cancer patients will present with advanced disease which is no longer operable and are often then incurable despite initial benefit from existing systemic treatments such as chemotherapy and or immunotherapy. Patients typically die from therapy resistant cancers that are widely spread in the body (metastatic disease). Therefore, understanding what makes lung cancer spread and become resistant to current treatments is critically important.
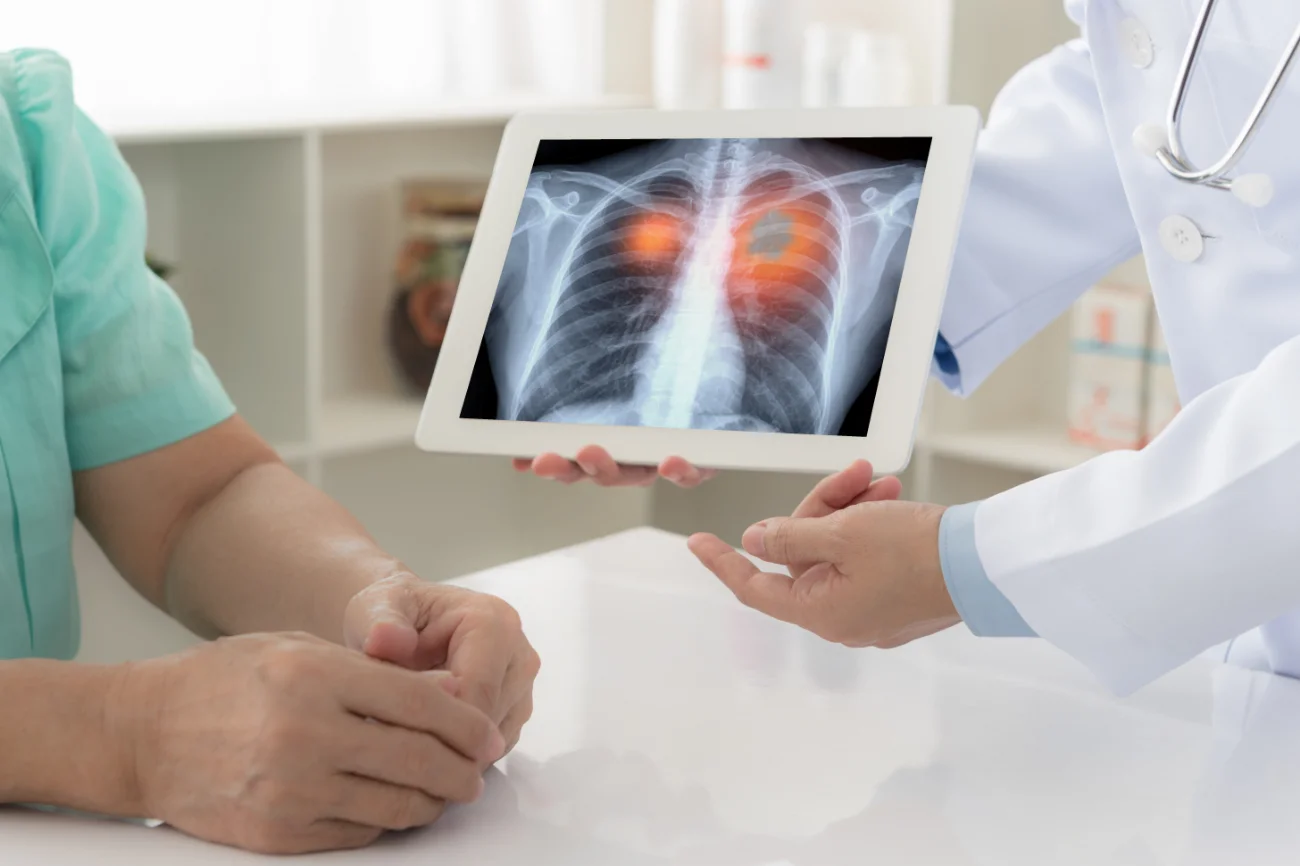
Our laboratories have had a long-standing interest in this research area, and we have recently discovered a new molecule inside lung cancer (and several other cancers) that is critically important in allowing the disease to spread and making the cancer resistant to existing treatments; such as chemotherapy, radiotherapy and immunotherapy. We have been dedicated to finding out if there is something we can do to reverse that effect, and our work has shown that an existing antibiotic that is used regularly to treat infection, targets this molecule and stops it from working.
The next stage is to develop a new drug that still takes out this target without being an antibiotic.
We are attempting to conduct a pilot study using the antibiotic to see if this will help patients, and we already have some primary data to suggest that this will be the case from prior work. The support from fundraisers would be gratefully received to help us continue to advance our work.
REFINE-Lung Immunotherapy Trial
We are conducting a large national NIHR funded immunotherapy study looking at reducing the frequency of giving pembrolizumab in lung cancer patients after 6 months of standard therapy (REFINE-Lung). Normally, patients receive this agent every 6 weeks for 2 years, but data from our prior work in gestational trophoblastic cancers has shown that just 6 months of treatment is sufficient. In addition, we know that a single dose of pembrolizumab can still be found stuck to its target on immune cells 200 days later. This information suggests that we could shorten the duration and/or reduce the frequency of giving pembrolizumab. Reducing the frequency after 6 months was acceptable to patients whilst early stopping was felt to be too risky. This trial has now been successfully recruiting patients and passed its interim safety analysis showing that we are not harming patients by giving the treatment less frequently. As immunotherapy does not work in every patient regardless of the frequency of giving it, a crucial issue is how to best identify patients who will fail therapy and might better receive alternative treatment. The CTRT is supporting research into addressing this question so the right treatment is given to the right patient at the right time.




